Release date: 2018-05-04
Professor Sun Yingxi, Professor of Reproductive and Genetic Specialist Hospital of the First Affiliated Hospital of Zhengzhou University, researcher and researcher of the Department of Life Science of Tsinghua University, and researcher of the Institute of Medicine, is the author of this article, the cooperative laboratory and the Li Wei researcher group of the Institute of Zoology, Chinese Academy of Sciences. Dr. Xu Jiawei and Dr. Yao Guidong from the First Affiliated Hospital of Zhengzhou University, Ph.D., Ph.D., Ph.D., Ph.D., Liu Biaofeng, Ph.D., Lin Zhefeng, Ph.D., and Ph.D. student of the medical school, Wang Peizhe, the co-first author of this article. The research was supported by the National Key Research and Development Program, the National Natural Science Foundation, and the China Youth Organization's Youth Thousand Talents Program.
Studies using mouse as a model organism showed that during the reprogramming of embryonic chromosomes, the open state of the source and the parental chromosome, the high-level structure and the epigenetic information carried by it were changed drastically. These changes can help mediate the initiation of embryonic genome transcription, reshape new pluripotent embryos, and lay the foundation for late embryonic development and cell differentiation. Previous studies have found that key regulatory elements of gene transcription are usually located in open areas of chromatin. These regulatory elements, together with cell-specific transcription factors, regulate cell fate determination and individual development.
Sun Yingxi's research team found that a large number of chromatin open regions were found in zygote, 2Cell, and 4Cell before human embryonic zygotic genomic activation (ZGA). The researchers first identified important transcription factors that may be involved in embryonic development, and further inhibited them. Embryo genome activation studies have found that early chromatin open regions rely on ZGA, and humans and mice have a conservative chromatin opening change in embryonic development. The researchers suggest that the open chromatin region unique to this early embryonic genome may serve as a special chromatin harbor ("chromatin harbor") that temporarily stores transcription factors that are turned off once the embryonic locus is activated. Transcription factors can be released into the promoter region to participate in genome activation. This pattern of chromatin changes that are conserved in human and mouse embryo development may play an important role in genomic silencing of early embryos and subsequent zygote genome activation. In addition, the researchers also studied the chromatin opening of two human embryonic stem cells (naïve hESC and primed hESC) and found that naïve human embryonic stem cells are closer to the inner cell mass of the human early embryo. This work has important reference value for studying human pluripotent cells in vivo and in vitro, and provides a theoretical basis for clinical application of stem cells. So far, the team research revealed the reprogramming of chromatin during early human embryo development, and deepened the understanding of chromatin status and gene expression regulation during embryonic development.
This study reveals the chromatin state and gene expression regulation patterns in the early development of human embryos. It reveals for the first time that there is a wide chromatin open region in human embryo ZGA, and clarifies its reprogramming pattern during embryonic development, expounding the transcriptional activation of embryo genome. The need for reprogramming of open chromatin regions. The research results provide a theoretical basis for understanding the epigenetic regulation of early development of human embryos, which will have a profound impact on the clinical development of assisted reproductive technology, and will be of great significance for improving the success rate of assisted reproduction and prevention and control of development-related birth defects.
In recent years, Professor Sun Yingwei of the First Affiliated Hospital of Zhengzhou University has made breakthroughs in the field of reproductive genetics and epigenetics: in response to the problem of repeated abortion in clinical chromosomal translocation patients, the allele mapping has been developed in conjunction with Yikang gene. Identification of embryonic horizontal chromosomal translocation carrying state technology (MaReCs), a novel preimplantation genetic diagnosis strategy, is expected to rewrite the birth outcome for tens of millions of chromosomal translocation carriers around the world, with relevant results in the Proceedings of the National Academy of Sciences ( PNAS) has been published and has been fully promoted to the clinic for the benefit of patients. In the field of RNA epigenetics research, Professor Sun Yingwei and the team of researcher Yang Yungui of the Beijing Institute of Genomics of the Chinese Academy of Sciences and the team of researchers of the Chinese Academy of Sciences Wang Hailin established a single base resolution RNA m5C. Sequencing and information analysis techniques, the first mRNA m5C modification map of mammals was mapped, and the first RNA m5C binding protein (Reader) ALYREF was identified and its function of regulating RNA nucleation was elucidated. The related results were studied in the Nature publication cell ( Cell Research) published as a cover story,
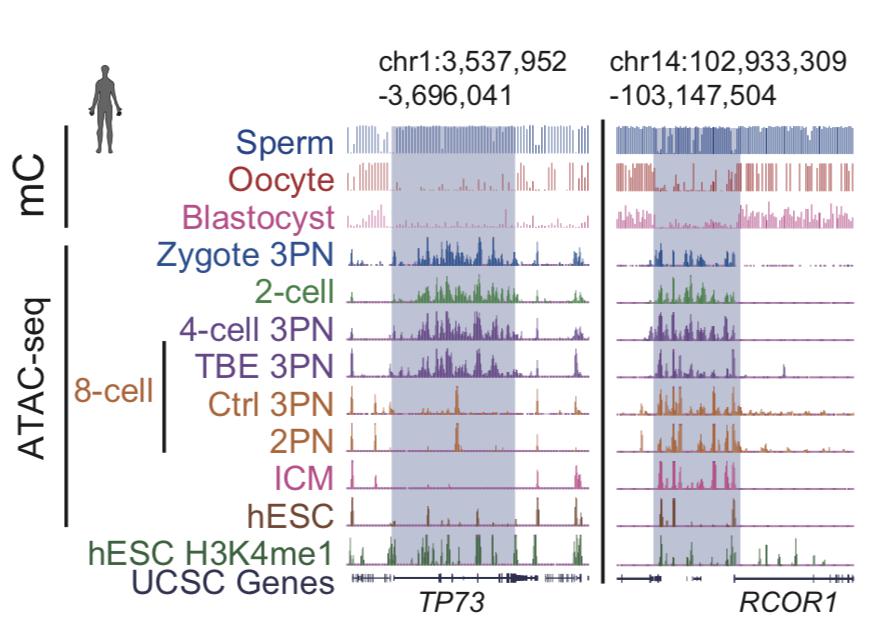
Chromatin open state at various stages of human embryonic development
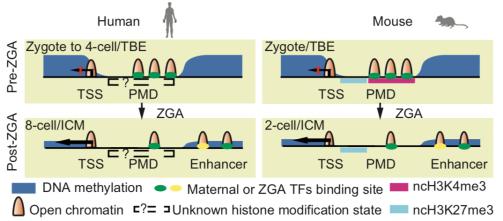
Early embryonic development chromatin open pattern
Source: Zhengzhou University
Nuts Seed Kernel include walnut kernel, sunflower seeds kernel, sunflower seed kernel etc., which are excellent food with rich nutrition.
Seed Kernel,Kernel Fruit,Walnut Kernel,Pumpkin seed kernel, sunflower seed kernel
Xi'an Gawen Biotechnology Co., Ltd , https://www.agolyn-bio.com