Model #: FG 2015
I.Treatment principle:
The Q-switched ND YAG laser takes specific wavelengths light in high energy, which are absorbed by the pigment and shatters the pigment into particles, breaking them into very small fragments , some parts will
consequently bounce out of the skin and the other parts will spilt even further into minute particles; which eventually will be engulfed by the phagocytes and ultimately gets eliminated by the lymphatic system.
II.Application:Â
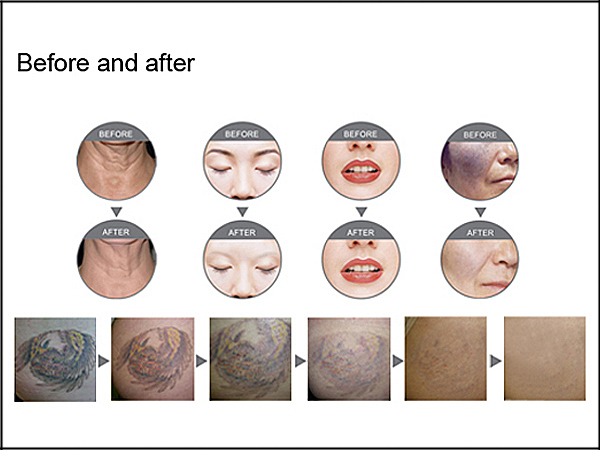
Pigment deposit dispelling
Color tattoo removal
Eliminates the age pigment, spot, birthmark
Pigment changes
Skin rejuvenation
Â
III.Advantages:Â
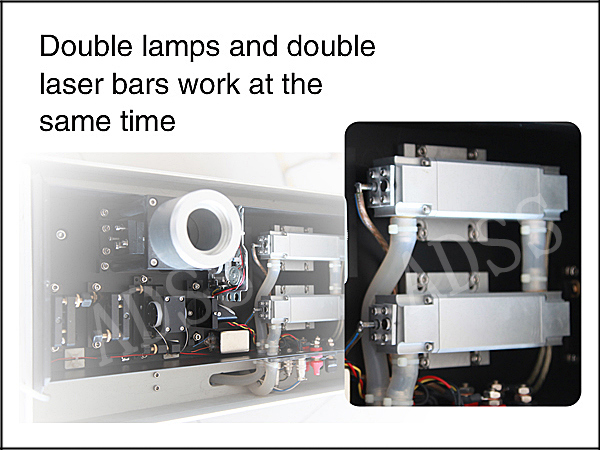
1.      Electro-optic Q-switch Nd Yag Laser, releases actual single pulse of laser energy. Really achieve to painless treatment and micro-injury to skin, long-term lasting treatment result
2.      Single pulse energy 1000mJ
3.      Professional for skin rejuvenation
Â
IV.Machine Features:Â
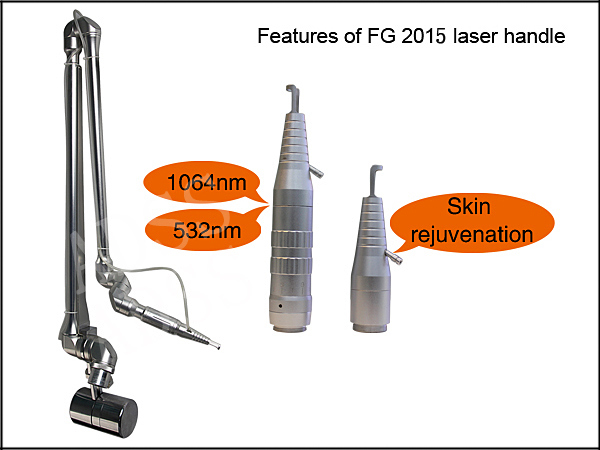
1. Spot size: 1-7mm adjustable
2. Excellent 7 articular-arm, to ensure the accuracy of long treatment and easy the operator's hand greatly
3. Alarm protection system of water flow and water temperature: protect people and machine against any risk at the first time
Â
V.Specifications:
| Laser type | Electro-optic Q-switch Nd Yag Laser |
| Wavelength | double wavelength 1064nm, 532nm |
| Controller | 8.4" TFT true color touch LCD |
| Single-pulse Energy | 1000mJ (1064nm); 500mJ (532nm) |
| Power | 2000W |
| Width of pulses | 6-12ns |
| Frequency | 1-10 |
| Diameter of spot | 1-7mm adjustable |
| Beam of light transmission | 7 articular-arm of light guide transmitting, transmission power is more than 75% |
| Indicator of aiming light | red semiconductor aiming light, wavelength 650-670nm |
| Cooling manner | closed-off water circulation + air |
| Dimension of machine | 83*32*83 cm |
| Dimension of package | 100*54*100 cm |
| N.W. | 60kg |
| G.W. | 102.5kg |
| Packing | wooden case |
| Voltage | 220V / 110V |
Quick details:Â
| Manufacturer | Yes, since 2005 |
| OEM&ODM | Yes, we have a professional research & development group of 20 people |
| Clinic training | offer |
| After-sales service | offer |
| Type | Stationary |
| Certificate | CE |
| Brand name | ADSS |
| Place of Origin | Beijing China (Mainland) |
| Packaging Detail | Wooden case |
| Delivery Detail | 3-7 DAYS |
| Transportation | By express (DHL, EMS, UPS, TNT, Fedex); By air express to air port;Â By sea |
| Payment method | T/T, WESTERN UNION, MONEY GRAM, ESCROW, PAYPAL |
Beijing ADSS Development Co., Ltd. can keep you always ahead in the aesthetics & medical laser machines field. ADSS has its own research & development, clinic, sales and after-sales departments; can offer the professional technology supports and clinic data at the first time. We are one of the most leading & reliable manufacturers in China, have a professional team integrating with optics, machinery, electricity and medicine that keep us ahead on in this field.
Â
We've developed into a modernized company, which covers 8,000m2 and has 200 more employees. Also we establish the branch company in Hong Kong and office in Japan. With efforts, ADSS company gained a number of domestic for his different equipments, and has the right of import and export certificate and the certificate of self-registration units' record management.Â
With the customer-orientated business philosophy and the purpose of science and technology first, ensure high quality and cost-effective products for customers; which makes us gains lots of customers all over the world. ADSS company works hard at all times, is to became a famous international OEM/ODM manufacturer of all aesthetic & medical equipments in the world. Â Â
Diagnostic reagents can be divided into two categories: in vivo diagnostic reagents and in vitro diagnostic reagents. It is mostly a reagent for detection by the reaction between antigen and antibody.
A: Classification of in vitro diagnostic reagents:
1. In vitro biodiagnostic reagents managed as drugs include:
1. Blood type and tissue type reagents;
2. Microbial antigen, antibody and nucleic acid detection reagents;
3. Tumor marker reagents;
4. Immunohistochemistry and human tissue cell reagents;
5. Human genetic testing reagents;
6. Biochips;
7. Allergy diagnostic reagents.
2. In vitro reagents managed as medical devices include:
1. Clinical basic test reagents;
2. Clinical chemistry reagents;
3. Blood gas and electrolyte determination reagents;
4. Vitamin determination reagents;
5. Cell histochemical stains;
6. Autoimmune diagnostic reagents;
7. Microbiological test reagents.
B: According to medical test items, clinical diagnostic reagents can be roughly divided into clinical chemical test reagents, immunology and
Serological testing reagents, hematological and cytogenetic testing reagents, microbiological testing reagents, body fluid excretion
Detection reagents, genetic diagnosis reagents, etc. Among them, the market share of clinical chemistry
The largest, close to 34%; followed by the immunology market, accounting for about 29%. Novel immunodiagnostic reagents and genetic diagnostic tests
The reagent was developed in the late 1980s, and it is the most common diagnostic reagent for all current diagnostic reagents, regardless of technology or market.
The fastest growing product.
Urine Rapid Test Kit,Rapid Test Kit 6-Panel,Toxoplasma rapid test kits,Fecal Occult Blood Test
Jilin Sinoscience Technology Co. LTD , https://www.jlgkscience.com