Medical Network October 24th Recently, CDE has released a new batch of public notices to be included in the priority review process drug registration application, which is the 33rd batch list since December 2015. On December 21, 2015, CDE released the first batch of applications for registration of children's drugs for priority review and approval, which was a hot topic in the industry. The national level made a determination for the development of children's drugs, and also gave children's drug manufacturers. New hope has come.
Minnet has sorted out 33 batches of proposed priority review lists. A total of 63 products in 33 batches (calculated by "product name + application company") are used for children, and children's drugs that were successfully included in the final batch. There are 57.
Figure 1: Review of children's drugs in the 33 batch priority review list (unit: unit)
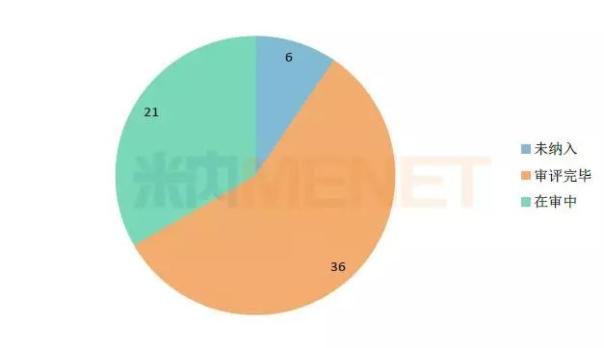
   (Source: Mnet China MED China Drug Evaluation Database 2.0)
As of October 22, 2018, 36 of the 57 children's medicines successfully included in the priority review have been reviewed, and 18 of them have been successfully approved (including approval of production, approval of imports, approval of clinical), and the remaining 18 The review results of the products are temporarily or otherwise. Of the 18 successfully approved products, 16 were new and 2 were generic.
16 children's new drugs were successfully approved: 7 new drugs, 9 new drugs
From the application point of view, among the 57 products that were included in the priority review process, there were 46 products for applying for new drug listing and new drug clinical trials. Currently, 33 products have been completed for the review and approval process, and the review results are approved for production. There are 16 products approved for approval and approved for clinical use.
Table 1: Status of 7 children's drugs approved for new drugs
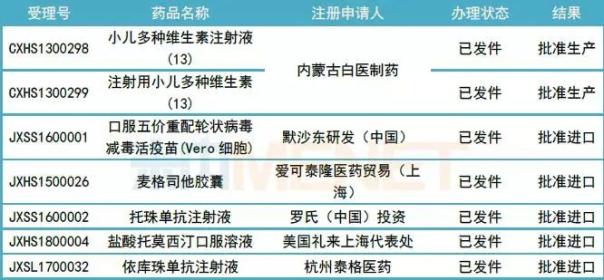
(Source: Mnet China MED China Drug Evaluation Database 2.0)
Among the 7 approved children's medicines for new drugs, 2 are domestically produced new drugs, all from Inner Mongolia Baiyi Pharmaceutical Co., Ltd. Pediatric multivitamin injection (13) and multi-vitamin (13) for injection can fully supplement all essential vitamins in children. The other 5 are imported new drugs, of which Megstatol capsule is used to treat rare type C Niemann's disease; totizumab injection is suitable for systemic juvenile idiopathic arthritis; eculizumab injection For the treatment of rare diseases in adults and children with paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
  Table 2: 9 children's drugs clinically approved for new drugs
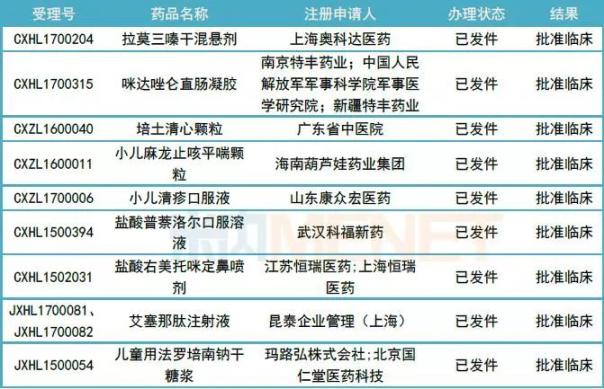
(Source: Mnet China MED China Drug Evaluation Database 2.0)
Among the 9 newly approved children's new drugs, 7 are domestically produced new drugs, 3 of which are proprietary Chinese medicines, namely Peitu Qingxin Granules, Xiaoer Malong Zhike Pingchuan Granules and Pediatric Qingyao Oral Liquid.
The other two are imported new drugs, exenatide injection is used to improve glycemic control in patients with type 2 diabetes, and currently listed exenatide injection has not been determined to be safe and effective in children, this approval The patient population for clinical studies is children.
Faropenem is an atypical β-lactam antibiotic belonging to penicillamine derivatives. Its mechanism of action is to block the synthesis of bacterial cell walls and bind to penicillin-binding protein (PBP) to exert a bactericidal effect. The approved clinical use of children's lyophilized sodium syrup is a child-specific dosage form for this product.
2 generic drugs were approved for approval: 1 approved for production and 1 approved for clinical
From the point of view of the application, there are 10 products for 57 generic products to be listed in the priority review process, and there are 2 products that have completed the review and approval process.
  Table 3: Status of approved children's generic drugs

(Source: Mnet China MED China Drug Evaluation Database 2.0)
Montelukast sodium particles main competitors on the market are Merck, in 2017 the city public hospital products in China, sales of county-level public hospitals, urban community centers and township hospitals (referred to as the Chinese public medical institutions) terminal of more than 1.8 100 million yuan. This product is suitable for the prevention and long-term treatment of asthma in children over one year old, including prevention of asthma symptoms during the day and night, treatment of aspirin-sensitive asthma patients and prevention of exercise- induced bronchoconstriction, and also for alleviating symptoms caused by allergic rhinitis ( Seasonal allergic rhinitis and perennial allergic rhinitis in children between 2 and 5 years of age).
At present, aripiprazole in the domestic market has only tablets and capsules, and there is no oral solution dosage form. Aripiprazole oral solution was jointly developed by Bristol-Myers Squibb and Otsuka Pharmaceuticals and approved by the FDA in 2005.
In addition to new drugs, generic drugs, there is also a supplement application, which is Sanofi's insulin glargine injection, which is for children and is suitable for type 1 diabetes. Currently, the supplementary application has completed the review and approval process, and the result is temporary. no.
21 children's drugs are still on the way to review: 8 new drugs to be listed are worthy of attention
Up to now, 21 children's medicines are still under review and approval, including 13 new products for new drugs and 13 new drugs for generic drugs.
Table 4: 13 new drug trials in children

(Source: Mnet China MED China Drug Evaluation Database 2.0)
Among the 8 children's medicines that apply for new drugs, 4 are domestic products, all of which are biological products, including 13-valent pneumococcal polysaccharide conjugate vaccine, group A C group meningococcal polysaccharide conjugate vaccine, nasal spray freeze-dried flu attenuated Live vaccine, recombinant human growth hormone injection. Among them, the 13-valent pneumococcal polysaccharide conjugate vaccine on the market is currently only produced by Pfizer, and the nasal spray freeze-dried influenza attenuated live vaccine has not been produced by enterprises.
  Table 5: 8 cases of children's generic drugs in trial

(Source: Mnet China MED China Drug Evaluation Database 2.0)
Source: CDE official website, Mene network database
Paeonia Albiflora Extract
Spine Date Seed Extract
Bitter Melon Extract
Mulberry Leaf Extract
Kudzu Root Extract
Dong-Quai/Angelica Extract
Ginger Extract
Epimedium Extract
Tribulus Terrestris Extract
Hawthorn Extract
Buck Wheat Extract
Siberian Gingseng Extract
Artichoke Extract
plant extract
Shaanxi HuiKe Botanical Development Co.,Ltd , https://www.oasis-hk.com