Medical Network March 8th
Abstract abstract
There are also a number of CAR-T therapy drugs accepted by CDE
Four companies submitted imitation of tenofovir disoproxil fumarate tablets
Merck's Pabloizumab Injection (PD-1 Monoclonal Antibody) is available for marketing
CFDA announces the second batch of generic drug approval evaluation announcement
Paclitaxel (albumin-binding) first imitation drug for injection was approved for production
Undertaking
According to the statistics of MED China Drug Evaluation Database 2.0, in February 2018, CDE had a total of 578 applications for drug registration . In February, CDE's application for drug registration was not affected by the Spring Festival holiday, and the application for the application increased in January.
Figure 1: Application for registration of drugs by CDE from September 2017 to February 2018 (according to the acceptance number)
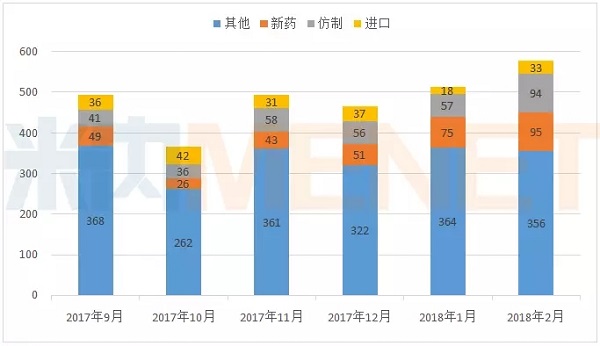
(Source: MED China Drug Evaluation Database 2.0, the same below)
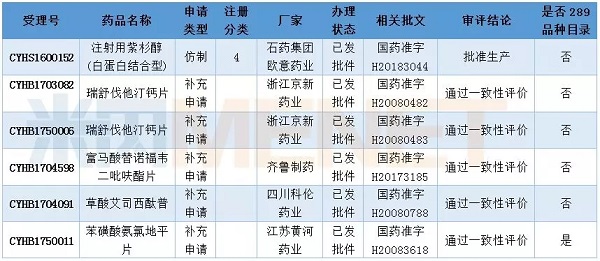
In February, 8 applications for consistency evaluation were undertaken by CDE, involving 7 varieties, of which metformin hydrochloride sustained-release tablets and irbesartan tablets belonged to non-289 catalogues.
Table 1: Acceptance of generic drug conformity assessment in February 2018
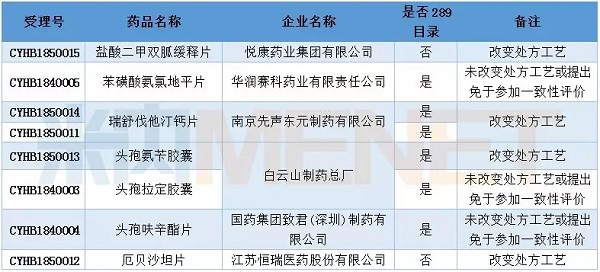
Domestic new drug contractor
There were 95 new drug applications in February, of which 63 were classified as Class 1 new drugs, involving 32 generic names, all of which were clinical applications. Chongqing Precision Bio, Shanghai Hengrun Dasheng Bio, Beijing Yimiao Medical Technology have a CAR-T therapy drug into the CDE, and Shanghai Youkadi Bio has two CAR-T therapy drugs in February. The company has three CAR-T therapies into the CDE. Since the first CAR-T therapy was approved by CDE in December 2017, more than 10 CAR-T therapies have been reviewed by CDE.
Table 2: Status of New Drugs in February 2018
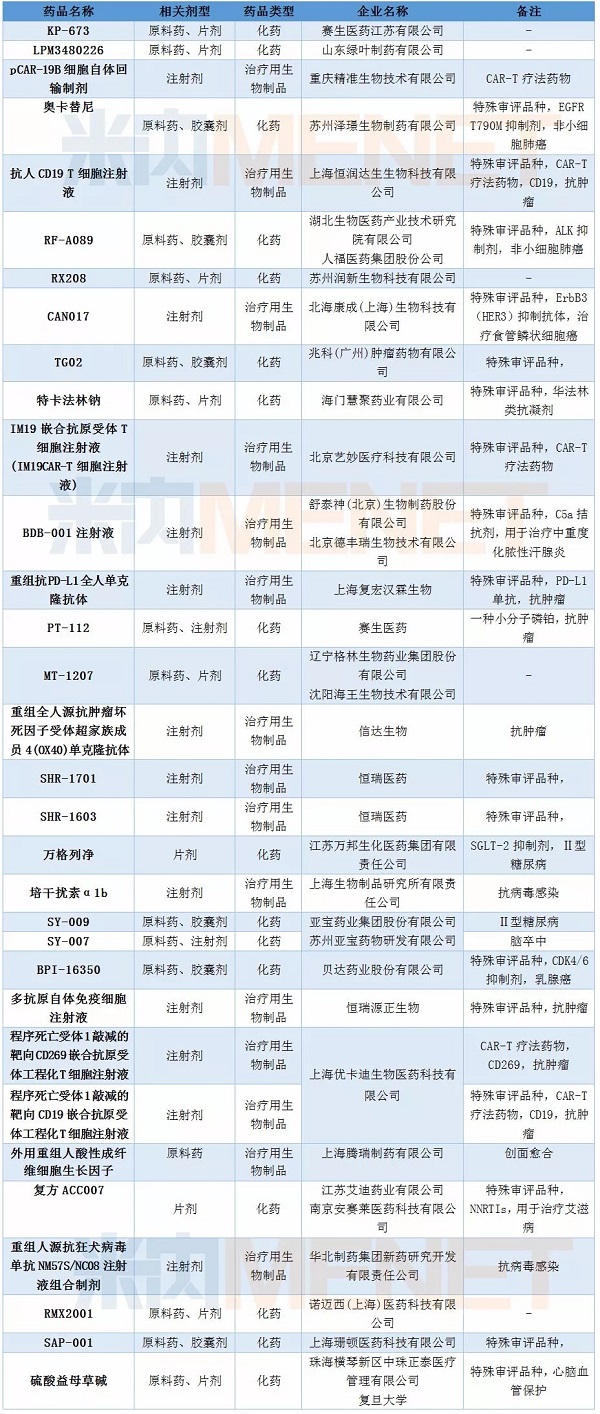
Domestic imitation contractor
In February, CDE hosted 94 generic applications, involving 53 varieties, of which 20 were currently exclusive domestic varieties. Iohexol injection, ambroxol hydrochloride injection and metformin hydrochloride sustained-release tablets have been approved in China and have signs of homogenization.
It is worth mentioning that in February, four companies applied for the imitation of tenofovir disoproxil fumarate tablets for CDE. As of the end of February 2018, two manufacturers have passed the evaluation of the generics of this variety. At present, there are 9 applications for imitation of this variety, and the competition is fierce.
Table 3: Application for imitation of February 2018
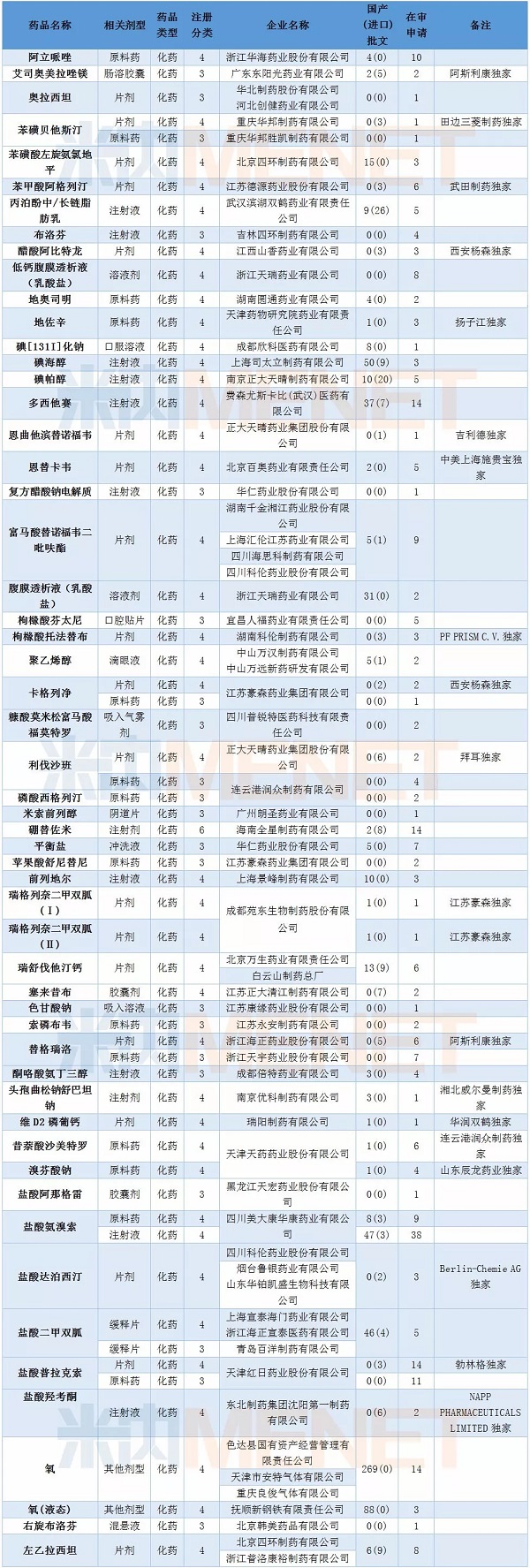
(Only the application for the trial is based on the type of new drug, imitation and import acceptance number)
Import application
In February, CDE hosted 33 import applications involving 21 varieties, 7 of which were the first for CDE. Merck's Pabolizumab injection, Roche's Alitinib Hydrochloride Capsule, and Jeter Bellin Human Albumin API are available for marketing.
Table 4: The registration of CDE import applications in February 2018

Approval status
In February, six varieties were evaluated through generic consistency. The injection of paclitaxel (albumin-binding type) of Shiyi Group Ouyi Pharmaceutical was first approved for production. The application is a new type 4 imitation application, which is regarded as passing the consistency evaluation, and the variety is also the second to pass the consistency evaluation. The injection (Sichuan Huiyu Pharmaceutical's injection of pemetrexed disodium is the first injection to pass the consistency evaluation).
In addition, CFDA announced the second batch of announcements on the consistency of quality and efficacy evaluation of generic drugs in February. There are 5 varieties in this time. Jiangsu Huanghe Pharmaceutical Co., Ltd., the subsidiary of Fosun Pharma, became the first to pass amlodipine besylate. Qilu Pharmaceutical became the second manufacturer to evaluate the consistency of tenofovir disoproxil fumarate tablets after Zhengda Tianqing.
Table 5: Approval of partial applications in February 2018
Hydrophilic Introducer Sheath Kit
Hydrophilic Introducer Sheath,introducer sheath,Hydrophilic,arterial sheath introducer
Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesia.com