"Dangdang jingle bells, we ski more happy, we are sitting on the sled..." With a cheerful song, another Christmas is coming, today's Christmas Eve, are you ready for long? Christmas stockings, come to the gift of your favorite? Don't worry, Santa Claus has already set off on a snowmobile with gifts. I believe that there are nine reindeer escorts that will surely capture all the monsters and ghosts and give you a big surprise at Christmas.
Closer to home, we all know that Tang Yan has a Sun Wukong all the way to demon escort, then who protects our body? When we are suffering from cancer, in addition to traditional surgery, chemotherapy, radiotherapy, what other generations of abilities can show great power? Is it PD-1/PD-L1, CAR-T or TCR ? Today, Xiaobian wants to bring you one of the Saints who conquered the tumor: CAR-T .
Conceptual analysis
CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) is a chimeric antigen receptor T cell immunotherapy [1] . This is a new type of cell therapy that has been around for many years, but has been improved and used clinically in recent years. It has significant efficacy in the treatment of acute leukemia and non-Hodgkin's lymphoma and is considered to be one of the most promising treatments for malignant tumors.
From 2010, CAR-T technology began its first clinical trial until 2017, when pharmaceutical giant Novartis Kymriah became the first CAR-T therapy approved by the US FDA . This therapy is used to treat acute lymphoblastic leukemia (ALL) in children and young adults (2 to 25 years old).
Shortly afterwards, the FDA announced that it has approved the listing of GATE 's CAR-T therapy, Yescarta, which is the second FDA-approved CAR-T therapy for the treatment of relapsed or refractory children and young adult B-cell acute lymphocytes. Treatment of leukemia [2] . Similar to other immunotherapies, its basic principle is to use the patient's own immune cells to clear cancer cells, but the difference is that this is a cell therapy rather than a drug therapy.

Figure 1. Emily Whitehead, the world's first leukemia girl to receive CAR-T treatment. In 2012, she participated in the CAR-T clinical trial after a second illness and now lives a happy and healthy life.
Like all technologies, CAR-T technology has undergone a long process of evolution. It is through this road that CAR-T technology can gradually mature.
CAR-T evolution process
Five generations of CAR-T cells have been designed so far (Figure 2). The generation of each generation is like an upgrade of the game in the game, a period is higher than a paragraph, a generation is stronger than a generation.
The first generation of bronze segments : The T cells in the first generation of CAR were activated using the CD3ζ intracellular signaling domain (first signal). However, these CARs do not produce sufficient IL-2 and have limited activation, amplification and anti-tumor effects in vivo.
Second generation silver segment : Generation of second generation CARs by the addition of costimulatory molecules with intracellular costimulatory signaling domains, such as CD28, CD137 (4-1BB), CD134 (OX40), CD27, ICOS and CD244. Compared with the first generation, the second generation of CAR produced 20 times more IL-2 and was able to continue to proliferate and kill target cells in vivo.
Third-generation gold segment : The third-generation CAR is produced by the addition of two or more costimulatory molecules with intracellular costimulatory signaling domains. Therefore, when more cytokines are secreted, the killing ability in vitro is enhanced. However, this has not achieved better results in the clinic.
The fourth-generation platinum segment: CAR is called T cell-redirected universal killer cytokine (or CAR) and is produced by adding a series of cytokines (such as IL-12), which can release cytokines locally. To regulate the microenvironment and recruit immune effector cells to kill tumors.
The fifth generation of diamond segments: The fifth generation of CAR-T is also based on the second generation, adding co-stimulatory domains that activate other signaling pathways, such as the intracellular binding of SAAT3/5 to IL2-2Rβ.
This generation and generation of different structural designs have given CAR-T cell therapy unlimited vitality and future. Although it has only been upgraded to five generations of diamonds, I believe that there will be six generations of Xingyao and seven generations of kings in the near future... We are waiting for it.

Figure 2. Evolution of CAR-T
Figure 2.Structure of different chimeric antigen receptor (CAR) generations. a The core structure of a CAR, highlighting the major components of the extracellular domain, the transmembrane domain and the intracellular domain (endodomain). b Evolution of the development of CARs from The first generation, which contained only ITAM motifs in the intracellular domain. Second-generation CARs included one co-stimulatory molecule (CM)1, and third-generation CARs contained a second CM. The fourth generation of CARs was based on second-generation CARs (containing 1–3 ITAMs) paired with a constitutively or inducibly expressed chemokine (eg IL-12). These T cells are also referred to as T cell redirected for universal cytokine-mediated killing (TRUCKs). The fifth, or 'next Generation', is also based on the second generation of CARs, with the addition of intracellular domains of cytokine receptors (eg IL-2Rβ chain fragment). ITAM immunoreceptor tyrosine-based activation motifs, CD co-stimulator y domain, IL-12 activation of interleukin 12 transcription, IL-2Rβ truncated intracellular interleukin 2β chain receptor with a STAT3/5 binding motif [3] .
Side effects of CAR-T
As the saying goes, "It is a three-point drug," and CAR-T is no exception. In 2006, TeGenero's anti-CD28 monoclonal antibody TGN1412 was tested in Phase I clinical trials. A total of 8 subjects, 2 of whom received placebo, 6 received real drugs, less than half an hour, 6 new drugs were tested. All of them had serious adverse reactions such as dyspnea, palpitations, and swelling of the whole body. They were sent to the intensive care unit. Fortunately, no one died. These adverse reactions were later discovered due to the large expansion of T cells in the body, resulting in the release of high concentrations of uncontrolled cytokines . This phenomenon is known as cytokine release syndrome (CRS), also known as cytokine storm . Although the launch of Kymriah and Yescarta has given hope to many patients, however, as with TGN1412, CAR-T treatment can also cause CRS, a side effect that cannot be ignored.
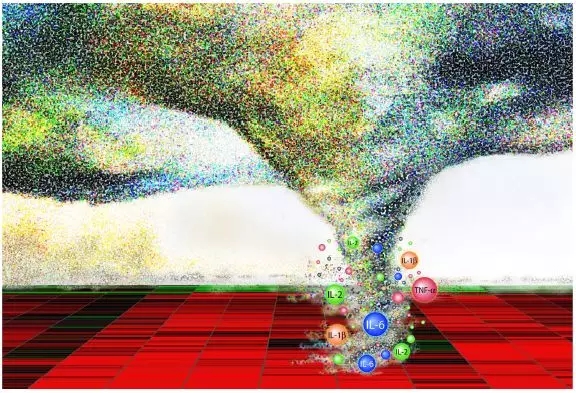
Figure 3. Cytokine storm [4]
Now that we have learned the basics of CAR-T, how does it apply to treatment?
CAR-T treatment process
A typical CAR-T treatment process is divided into the following five steps [5] .
The first step (separation) : Isolation of immune T cells from cancer patients.
The second step (modification) : a chimeric antibody capable of recognizing tumor cells and simultaneously activating T cells is added to T cells by genetic engineering techniques, that is, CAR-T cells are prepared.
The third step (amplification) : in vitro culture, a large number of expansion of CAR-T cells. In general, a patient needs billions or even billions of CAR-T cells (the larger the body, the more cells are needed).
The fourth step (return) : the amplified CAR-T cells are returned to the patient.
Step 5 (Monitoring) : Strictly monitor the patient, especially the violent reaction of the body a few days before the control.
The entire course lasts for about 3 weeks, in which the cell "extraction-modification-amplification" takes about 2 weeks and takes a long time.
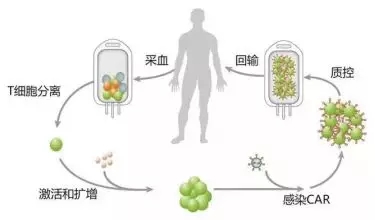
Figure 4. CAR-T treatment process [6]
Future prospects
CAR-T Cell Therapy is a very promising area of ​​research that represents the beginning of a new era of cancer treatment using engineered T cells and has unlimited potential for future treatment of all diseases. Although it has shown impressive results in clinical research, there are still many obstacles that hinder its wide range of applications, including the complex genetic technologies required to make CAR-T cells, tumor escape, inadequate toxicity management, and disease. Recurrence is relatively fast, lack of persistence, and the need for standardized CAR-T cell therapy [7] . Scientists from MIT and Boston University have developed a universal CAR-T cell system, the SUPRA CAR system, which includes a variety of upgrades, such as switching targets, T cells without reprogramming T cells. The degree of activation is completely controllable, and multiple antigens can be perceived and logically responded . These features can be used to combat tumor recurrence, eliminate over-activation and enhance the specificity of CAR-T cell therapy, which will be a direction for future development [8] . At present we only see the tip of the iceberg, and many key issues have not yet been answered. But the question will eventually be answered, and CAR-T will ultimately benefit mankind.
Ok, today Xiaobian will bring everyone here, and later will bring you more science knowledge, so stay tuned! ! !

Scan code to pay attention to the 100 Olympics map to learn more about consulting
Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.sntextract.com